R&D
- Feb 6, 2023
Iron-molybdenum composite wires for thin vascular or cerebrovascular devices
Adam J. Griebel, Jeremy E. Schaffer
Some implantable medical devices intended to treat maladies of the arteries of the brain, like flow diverters, require very thin wires, often 0.001” (25 µm) or less. Absorbable versions of these devices could offer several long-term advantages, but selecting a suitable material is challenging. Magnesium alloy wire at these sizes will likely degrade too quickly and be impossible to see with fluoroscopy. Zinc alloys are not elastic enough. Iron alloys, like our FeMnN alloy [1], may have an appropriate degradation rate but can still be prone to localized degradation, and would likely need radiopacity aids. Molybdenum (Mo) has recently been proposed as an absorbable metal [2] [3] and may offer a suitable corrosion rate and improved radiopacity, though much needs to be learned about its behavior in the body.
A new concept from Fort Wayne Metals R&D leverages our knowledge of absorbable metal alloy systems, our experience with producing DFT® wire composites, and our unique patent portfolio [4] [5]. We have produced several composite wires with FeMnN alloy shells and one or more Mo filaments embedded within. The galvanic relationship between FeMnN and Mo is such that the FeMnN will have its corrosion accelerated once the Mo is exposed, perhaps providing enough accelerant to overcome the passivating corrosion layer that plagues iron-based alloys. Based on prior work, FeMnN can provide enhanced elasticity and improved endothelial cell adhesion to promote reendothelialization [6, 7]. In turn, the Mo is galvanically protected from corrosion until the FeMnN is gone, providing a “programmable” staged degradation of the wire. Such wire might readily serve in absorbable flow diverters or other absorbable vascular devices.
Some of the prototype two-metal cross-sections produced are shown in Figure 1 below. Each of the cross-sections shows an outer shell layer of FeMnN surrounding one or an array of Mo core filaments. Trials to understand in vitro and in vivo degradation are underway, and work to characterize radiopacity is planned.
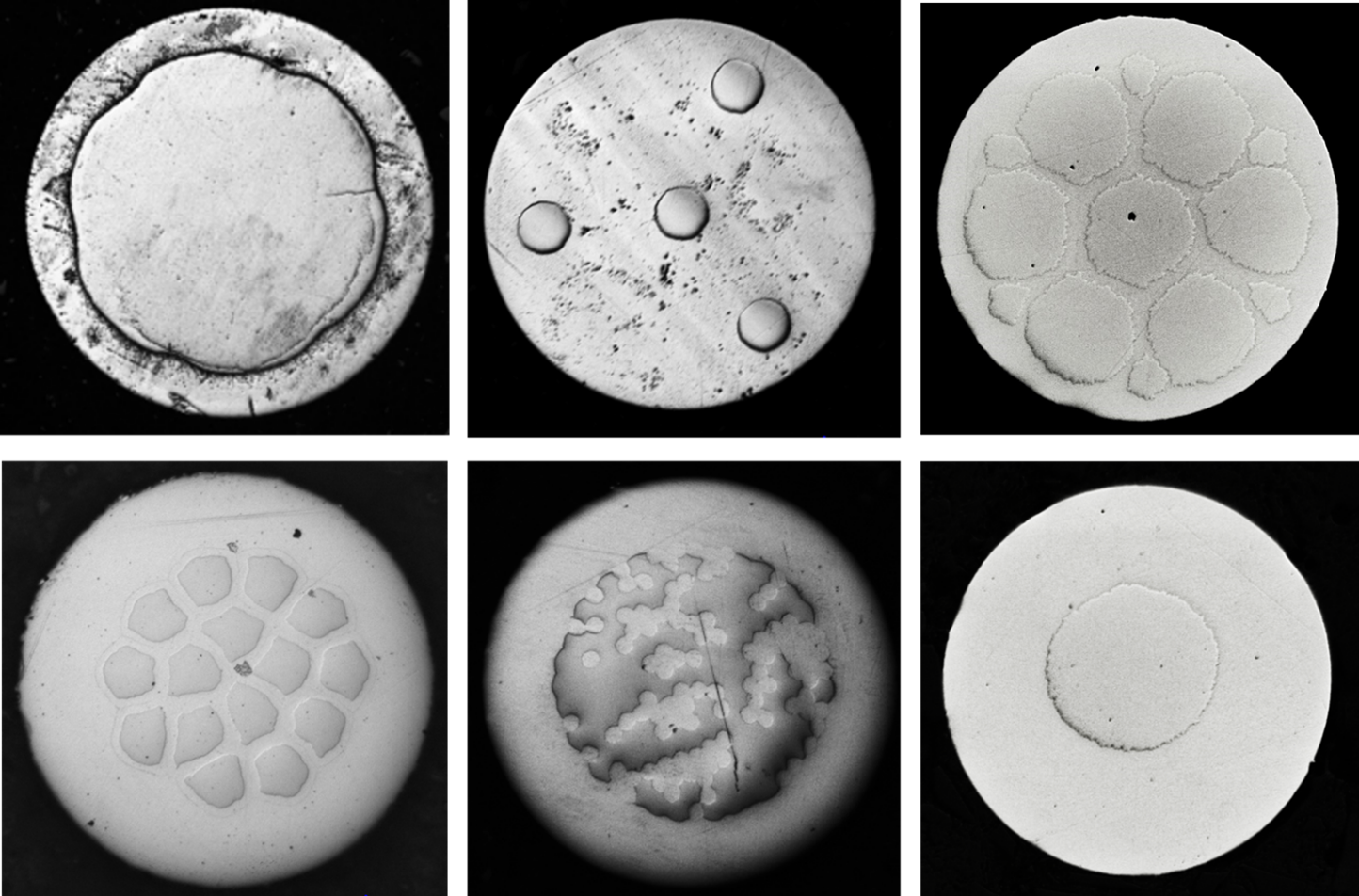
References
- [1] J. Schaffer, "Biodegradable alloy wire for medical devices". EU Patent EP2872663B1, 2020.
- [2] C. Redlich, P. Quadbeck, M. Thieme and B. Kieback, "Molybdenum – A biodegradable implant material for structural applications?," Acta Biomaterialia, vol. 104, pp. 241-251, 2020.
- [3] M. Sikora-Jasinska, L. Morath, M. Kwesiga and e. al, "In-vivo evaluation of molybdenum as bioabsorbable stent candidate," Bioactive Materials, vol. 14, pp. 262-271, 2022.
- [4] J. Schaffer, "Biodegradable composite wire for medical devices". USA Patent US9561308B2, 2017.
- [5] M. S. Michael, H.-J. Wachter and R. J. Myers, "Drawn strand filled tubing wire". USA Patent US7745732B2, 2010.
- [6] J. E. Schaffer, E. A. Nauman and L. A. Stanciu, "Cold-drawn bioabsorbable ferrous and ferrous composite wires: An evaluation of mechanical strength and fatigue durability," Metallurgical and Materials Transactions, vol. 43, no. B, pp. 984-994, 2012.
- [7] J. E. Schaffer, E. A. Nauman and L. A. Stanciu, "Cold drawn bioabsorbable ferrous and ferrous composite wires: an evaluation of in vitro vascular cytocompatibility," Acta biomaterialia, vol. 9, no. 10, pp. 8574-8584, 2013.
If you have questions, or want to know more about other projects and innovations, we'd like to hear from you. Please contact us: [email protected]
For more updates, visit our Library of Updates
Disclaimer: Our updates are sneak peeks of what our R&D department is working on. This is not meant to imply that we have what is referenced above ready for robust serial manufacture.